By Eric Scerri
This article is about how new elements are discovered and added to the periodic table. This is a process that has been occurring ever since the periodic table was first published in its mature form in 1869 by the Russian chemist Dimitri Mendeleev. In fact the reason why Mendeleev is generally credited with discovering the periodic table, in preference to several less known scientists, is because only he made predictions of new elements, which were subsequently discovered.

Dmitri Mendeleev

Henry Moseley
Mendeleev initially predicted that four new elements should exist as well as predicting a number of the properties he expected them to have. Within 15 years three of these four elements were discovered and named gallium, germanium and scandium. It soon emerged that their properties were remarkably similar to Mendeleev's predictions. Mendeleev's fame began to grow and to this day he is perhaps the most famous scientist to emerge from Russia.
Key elements discovered
In 1913 the English physicist Henry Moseley made the prediction of new elements more tractable when he established that the property of the atomic number, or the positive charge of any particular atom's nucleus, was the best way to order the elements in the periodic table. Moseley's work showed that precisely seven elements remained to be discovered. These new elements included protactinium, hafnium, rhenium, technetium, francium, astatine, and promethium. Whereas the first three of these occurred naturally, the remaining four were created artificially in particle accelerators. The first of these artificially synthesized elements was technetium that was created in 1947 and incidentally the fourth of Mendeleev's original predictions.
This element, or rather one of its isotopes Tc-99, now has an important application in medical imaging. The nuclear isomer of technetium is radio-active with a half-life of about six hours, a feature that makes it very suitable for use with human subjects because in a 24 hour period 94% of the isomer decays from the body.
But even before the periodic table flanked by hydrogen, the lightest element, and uranium the heaviest one, had been completed some scientists had succeeded in synthesizing an element beyond uranium. In 1940 McMillan and Abelson working in Berkeley, California created atoms of a new element that was named neptunium. Its position in the periodic table after Uranium, led to it being named after Neptune, the planet that comes after Uranus as one moves away from the sun.
In the 76 years since then a total of 24 further elements have been artificially created in various laboratories around the world including the US, Russia, Germany and Japan. The heaviest element of all is element number 118. Four of these elements, numbers 113, 115, 117 and 118 have recently been officially ratified and will soon receive official names. [ They were finally named as nihonium(Nh), moscovium(Mc), tennessine(Ts), and oganesson(Og), respectively ].
The most recently discovered of these super-heavy elements, as they are known, was element 117 whose discovery marked a unique moment in the history of the periodic table and the elements. It was the first time that the periodic table was complete in the sense that every space in every single row of the table contained a known element. Needless to say, the synthesis of element 118 does not necessarily spell the end of the periodic table and active efforts are currently being pursued to create elements 119 and 120.
How are new elements added to the periodic table?
But let me return to the question of incorporating newly found or synthesized elements into the periodic table because an interesting twist on this issue has arisen in recent years. When elements reach high atomic numbers their chemical behaviour becomes substantially modified by what are called relativistic effects. According to Einstein's 1905 theory of relativity, very fast moving objects undergo a number of bizarre effects including length contraction. In the case of electrons orbiting around the nuclei of atoms the effect of very high speeds is to contract the radius at which the electron motion takes place. This behaviour has a knock on effect of other electrons with the result that the chemistry of these atoms takes on an unexpected turn.
In the 1990s elements 104 and 105, rutherfordium and dubnium respectively, were synthesized. Although these elements are highly unstable, scientists were able to probe some of their chemical reactions and reach the surprising conclusion that they did not behave in the way that was expected of them. Whereas the cardinal rule of the periodic table is that elements falling in the same column of the periodic table show similar chemical properties. For example, instead of behaving like hafnium the element lying above it in the periodic table, rutherfordium was found to be more like plutonium which lies a considerable distance away from it, as can be seen from figure 2. [ Please note that Element 104, rutherfordium(Rf), was previously known as kurchatovium(Ku). And Element 105, dubnium(Db), was previously known as hahnium(Ha). And that the names of the elements 110, 111, 112, 114, 116 are darmstadtium(Ds), roentgenium(Rg), copernicium(Cn), flerovium(Fl), and livermorium(Lv), respectively ].
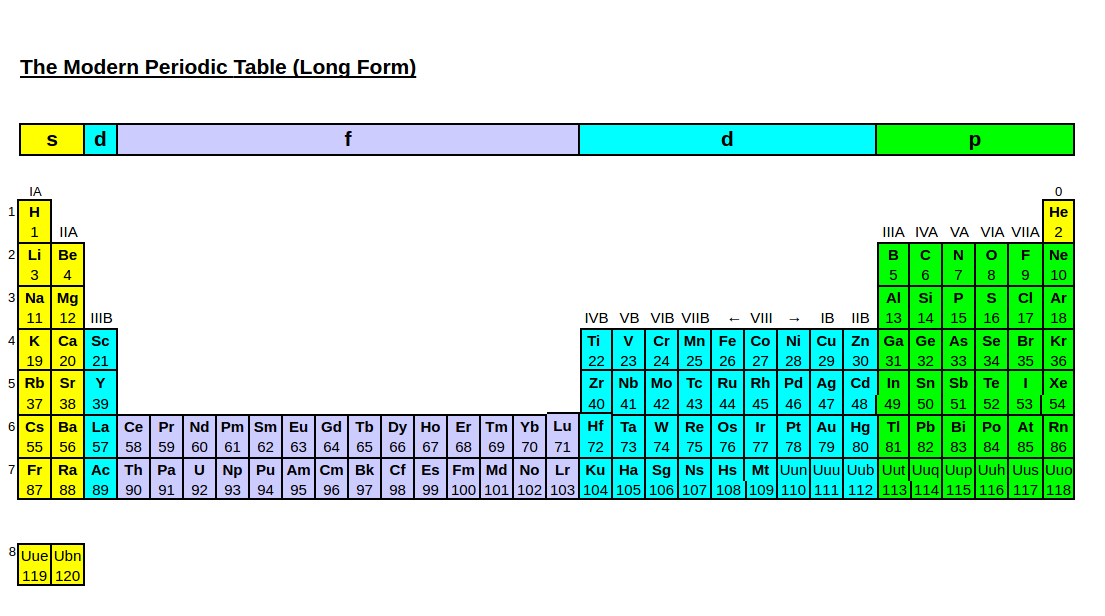
This version of the periodic table displayed, in its 32-colum or long form, incorporates the so called f-block elements in a natural manner rather than displaying them as a disconnected footnote below the main body of the table.
Is the periodic table beginning to show cracks?
Some experts reacted by concluding that the periodic table and our ability to incorporate the elements had begun to show 'cracks'. It was beginning to seem as if relativistic effects were playing havoc with the much prized periodic table. The situation today is far from resolved. When element 107, called bohrium, was synthesized, there were expectations that it too might show anomalous chemical properties. However all experiments conducted on the new element confirmed that it belonged firmly below the element rhenium. The reliability of the periodic table had made a quick 'come back' and the discoverers of the new element entitled their article "Boring Bohrium" in order to signal that there was no unexpected behavior that might have been expected on the grounds of what had been found in elements 104 and 105.
But the notion that relativity plays havoc with the chemist's periodic table has not yet been laid to rest. In any case even if it turns out to be true, the periodic system will still maintain its status as the over-arching organizing principle which is used to understand the properties of all but the most unstable elements and materials that are made up of the stable elements.
Dr. Eric Scerri is in the chemistry department at UCLA in Los Angeles and has authored several popular science books www.ericscerri.com
|
|
|